Which Of The Following Types Of Animals Lacks A Nervous System?
How does our nervous system operate so quickly and efficiently? The answer lies in a membranous construction called myelin.
All our activities — eating, walking, talking — are controlled past our brains, the center of the nervous organization. The brain receives huge amounts of information from exterior our torso via our v senses (vision, audio, taste, touch, and smell), integrates this information, and orders our muscles to take activeness. How is all that achieved so efficiently? The respond lies in a membrane structure called myelin.
Information Manual in the Body
All data both to and from the body must exist coordinated and transmitted simultaneously and very apace. The brain itself requires extremely fast speeds to operate at even at the simplest level. How practice the biological tissues of our trunk back up such rapid coordination of the brain, limbs, and sensory input? They practise so with nervous system tissue that imitates electrical wiring.
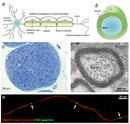
The nervous system is comprised of two chief cell types: neurons and glial cells. These cells communicate with each other to perform of import tasks in the nervous system. The glial cells support neurons structurally and maintain their long-term neuronal integrity, and neurons regulate glial cell behavior. In this support of neurons, glial cells have become highly specialized. Glial cells, which can exist divided into several types, have various important functions, such as providing structural support, growth support, and insulation effectually the axon.
Why must glial cells support neurons? Neurons are specialized cells that receive and transport signals to other cells through fragile and thin cellular extensions called axons. These axons extend over distances long and curt to attain their target, ultimately connecting neurons with other nervus tissue, muscle tissue, or sensory organs (Figure 1A). For example, some motor neurons in the spinal cord have axons that exceed 1 m in length, connecting the spine to the lower limb muscles. These axons transmit signals to the target muscle in the form of electric impulses chosen activeness potentials. Withal, the axons lone are not enough to produce rapid conduction of the electric current necessary for this signal to be sent. Glial cells are the primal chemical element for supporting the messages neurons send and receive all over the torso. Much similar the insulation around the wires in electrical systems, glial cells grade a membraneous sheath surrounding axons chosen myelin, thereby insulating the axon. This myelination, as it is chosen, can profoundly increase the speed of signals transmitted between neurons (known equally activeness potentials). Indeed, the evolution of myelin allowed vertebrates to attain efficient nervous systems despite their large body size.
The Myelin Sheath: Shape, Size, and Limerick
What exactly is myelin? Myelin is a concentrically laminated membrane construction surrounding an axon around which lamellae (or cellular protrusions) echo radially at a period of about 12 nm (Waxman, Kocsis & Stys 1995; Sherman & Brophy 2005). The myelin lamella is formed by fusion of the apposed inner leaflets of the plasma membrane in glial cells, with no intervening cytoplasm (Figure 1B).
Depending on the location, different glial cell types make myelin in a different manner. Schwann cells make myelin in the peripheral nervous arrangement (PNS: nerves) and oligodendrocytes in the central nervous organization (CNS: brain and spinal string). In the PNS, one Schwann cell forms a single myelin sheath (Figure 1A). Past contrast, in the CNS, the oligodendrocyte sends cell processes to myelinate multiple segments on many axons (Effigy ii). Although in that location are several molecular or morphological differences between nerve fibers in the PNS and CNS, the basic myelin sheath arrangement and the electrophysiological characteristics are essentially the aforementioned.
Are all axons covered with myelin? No; they tin can be either myelinated or unmyelinated. Myelinated axons are ensheathed forth their entire length. The axon caliber (diameter) in mammalian PNS ranges from 0.1 μm to 20 μm, with unmyelinated axons being less than 2 μm and myelinated axons being more than one–ii μm in bore. In the CNS, almost all axons with diameters greater than 0.2 μm are myelinated. In cross department, the myelinated axon appears every bit a nearly round profile surrounded by a spirally wound multilamellar sheath (Figure 1C and D). Amazingly, a large myelinated axon may take up to 250 to 300 turns of myelin wrapping around it. The ratio between axon diameter and that of the full nervus fiber (axon and myelin) is 0.six–0.7, a ratio that is well maintained regardless of the axon caliber. The length of the myelin sheath along the axon is approximately 1 mm in the PNS. Between 2 adjacent myelin segments, at that place are approximately 1-μm-long gaps called nodes of Ranvier (Effigy 1A and E). At the nodes, the axon is exposed to the extracellular space.
Axonal Signaling Regulates Myelination
How is the spiral wrapping of the myelin sheath around axons formed precisely and accordingly? One machinery has been identified in PNS myelination. In the PNS, neuregulin 1 type 3 protein is expressed on the axon surface and interacts with glial ErbB receptors, and it has a pivotal role for Schwann jail cell differentiation and myelination (for review, see Nave & Salzer 2006). Unmyelinated autonomic neurons express depression levels of neuregulin one type 3 on the axon surface, whereas heavily myelinated axons express loftier levels.
Without neuregulin 1 type Three, Schwann cells in civilisation derived from these mutant mice cannot myelinate neurons in the spinal cord (dorsal root ganglion neurons). Intriguingly, in unremarkably unmyelinated fibers, forced expression of neuregulin 1 type 3 in the postganglionic fibers of sympathetic neurons grown in civilisation tin exist forced to myelinate. Thus, the level of neuregulin i type Three on the PNS axons is a key instructive signal for myelination. Furthermore, above the threshold, the myelin formation is correlated with the amount of neuregulin i type Three presented by the axon to the Schwann cell. Reduced expression of neuregulin i type III leads to a thinner than normal myelin sheath in the heterozygous mutant mice of this molecule. In contrast, transgenic mice that overexpress neuregulin 1 become hypermyelinated.
One interesting question is: Does neuregulin-ErbB signaling regulate CNS myelination every bit well? Although several reports show that oligodendrocytes respond to neuregulin 1 in vitro, analyses of a series of conditional naught mutant animals defective neuregulin 1 showed normal myelination (Brinkmann et al. 2008). It is withal unclear how myelination is regulated in the CNS.
Myelin Promotes Rapid Impulse Transmission Along Axons
How does myelin enhance the speed of action potential propagation? Information technology insulates the axon and assembles specialized molecular structure at the nodes of Ranvier. In unmyelinated axons, the activity potential travels continuously along the axons. For case, in unmyelinated C fibers that conduct pain or temperature (0.4–ane.2 μm in diameter), conduction velocity along the axon is 0.5–ii.0 yard/south (as fast equally you walk or jog).
In contrast, amid the myelinated nerve fibers, axons are mostly covered by myelin sheaths, and transmembrane currents tin can only occur at the nodes of Ranvier where the axonal membrane is exposed. Myelin is rich in lipids (approximately lxxx%) and tin therefore act as an insulator (pregnant high transverse resistance and a low electrical capacitance) forth the internodal segments. For example, conduction velocity in the most thoroughly myelinated axons (12–twenty μm in diameter) is 70–120 m/s (race automobile speed), although other factors such equally axon caliber can influence this velocity.
At nodes, voltage-gated sodium channels are highly accumulated and are responsible for the generation of action potentials. To induce and maintain nodal sodium aqueduct clusters, specific molecules are also enriched at nodal axons, including cell adhesion molecules such equally neurofascin 186 and cytoskeletal and scaffolding proteins such as bIV spectrin (Poliak & Peles 2003; Susuki & Rasband 2008). The myelin helps gather this nodal molecular organisation. For example, during the development of PNS myelinated nerve fibers, a molecule called gliomedin is secreted from myelinating Schwann cells so incorporated into the extracellular matrix surrounding nodes, where information technology promotes assembly of nodal axonal molecules. Due to the presence of the insulating myelin sheath at internodes and voltage-gated sodium channels at nodes, the activity potential in myelinated nerve fibers jumps from ane node to the next. This manner of travel by the action potential is called "saltatory conduction" and allows for rapid impulse propagation (Effigy 1A).
Myelin Damage Causes Astringent Neurological Diseases
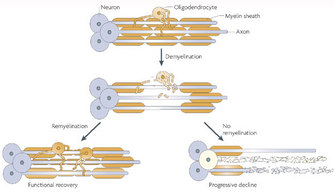
© 2008 Nature Publishing Group Franklink, R. J. M. & ffrench-Constant, C. Remyelination in the CNS: from biology to therapy. Nature Reviews Neuroscience 9, 839–855 (2008). All rights reserved. ![]()
What happens if myelin is damaged? The importance of myelin is underscored by the presence of diverse diseases in which the chief problem is defective myelination. Demyelination is the condition in which preexisting myelin sheaths are damaged and afterwards lost, and information technology is one of the leading causes of neurological illness (Figure 2). Primary demyelination tin can be induced by several mechanisms, including inflammatory or metabolic causes. Myelin defects too occur by genetic abnormalities that affect glial cells. Regardless of its cause, myelin loss causes remarkable nervus dysfunction because nervus conduction can be slowed or blocked, resulting in the damaged data networks between the brain and the body or inside the brain itself (Effigy 3).
Following demyelination, the naked axon tin be re-covered by new myelin. This process is chosen remyelination and is associated with functional recovery (Franklin and ffrench-Constant 2008). The myelin sheaths generated during remyelination are typically thinner and shorter than those generated during developmental myelination. In some circumstances, however, remyelination fails, leaving axons and even the entire neuron vulnerable to degeneration. Thus, patients with demyelinating diseases suffer from diverse neurological symptoms.
The representative demyelinating disease, and mayhap the most well known, is multiple sclerosis (MS). This autoimmune neurological disorder is caused by the spreading of demyelinating CNS lesions in the unabridged brain and over time (Siffrin et al. 2010). Patients with MS develop diverse symptoms, including visual loss, cognitive dysfunction, motor weakness, and pain. Approximately lxxx percent of patients experience relapse and remitting episodes of neurologic deficits in the early phase of the illness (relapse-remitting MS). There are no clinical deteriorations between two episodes. Approximately ten years after affliction onset, nearly one-half of MS patients suffer from progressive neurological deterioration (secondary progressive MS). About 10–fifteen percent of patients never experience relapsing-remitting episodes; their neurological condition deteriorates continuously without any comeback (chief progressive MS). Importantly, the loss of axons and their neurons is a major cistron determining long-term inability in patients, although the primary cause of the affliction is demyelination. Several immunodulative therapies are in employ to prevent new attacks; however, in that location is no known cure for MS.
Research in Myelin Biology and Pathology
Despite the severe outcome and considerable upshot of demyelinating diseases on patients' lives and guild, niggling is known near the machinery by which myelin is disrupted, how axons degenerate after demyelination, or how remyelination can be facilitated. To establish new treatments for demyelinating diseases, a better understanding of myelin biology and pathology is absolutely required.
How do we structure a research effort to elucidate the mechanisms involved in developmental myelination and demyelinating diseases? We need to develop useful models to test drugs or to modify molecular expression in glial cells. One strong strategy is to use a culture arrangement. Coculture of dorsal root ganglion neurons and Schwann cells can promote efficient myelin germination in vitro (Figure 1E). Researchers can alter the molecular expression in Schwann cells, neurons, or both past various methods, including drugs, enzymes, and introducing genes, and can discover the consequences in the culture dish.
Modeling demyelinating illness in laboratory animals is normally achieved by handling with toxins injurious to glial cells such as lysolecithin or cuprizone. Autoimmune diseases such as MS or autoimmune neuropathies can be reproduced by sensitizing animals with myelin proteins or lipids (Effigy three). Some mutant animals with defects in myelin proteins and lipids accept been discovered or generated, providing useful disease models for hereditary demyelinating disorders. Further research is required to understand myelin biological science and pathology in detail and to constitute new treatment strategies for demyelinating neurological disorders.
Summary
Myelin can greatly increase the speed of electrical impulses in neurons because information technology insulates the axon and assembles voltage-gated sodium channel clusters at discrete nodes along its length. Myelin damage causes several neurological diseases, such as multiple sclerosis. Future studies for myelin biology and pathology volition provide important clues for establishing new treatments for demyelinating diseases.
References and Recommended Reading
Brinkmann, B. Thousand. et al. Neuregulin-i/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous arrangement. Neuron 59, 581–595 (2008).
Franklin, R. J. & ffrench-Constant, C. Remyelination in the CNS: From biology to therapy. Nature Reviews Neuroscience 9, 839–855 (2008).
Nave, K. A. & Salzer, J. L. Axonal regulation of myelination by neuregulin 1. Current Stance in Neurobiology xvi, 492–500 (2006).
Poliak, Due south. & Peles, E. The local differentiation of myelinated axons at nodes of Ranvier. Nature Reviews Neuroscience 4, 968–980 (2003).
Sherman, D. L. & Brophy, P. J. Mechanisms of axon ensheathment and myelin growth. Nature Reviews Neuroscience vi, 683–690 (2005).
Siffrin, 5. et al. Multiple sclerosis — candidate mechanisms underlying CNS atrophy. Trends in Neurosciences 33, 202–210 (2010).
Susuki, K. & Rasband, M. Northward. Molecular mechanisms of node of Ranvier germination. Current Opinion in Cell Biology xx, 616–623 (2008).
Waxman, S. G., Kocsis, J. D. & Stys, P. K., eds. The Axon: Construction, Function and Pathophysiology. New York: Oxford University Press, 1995.
Source: https://www.nature.com/scitable/topicpage/myelin-a-specialized-membrane-for-cell-communication-14367205/
Posted by: huttonlecoany.blogspot.com

0 Response to "Which Of The Following Types Of Animals Lacks A Nervous System?"
Post a Comment